-
Постов
771 -
Зарегистрирован
-
Посещение
Тип контента
Профили
Форумы
Загрузки
Articles
Помощь
Весь контент Θðηα mάkαя
-
NOTE: This is a sponsored press release written by VAPORESSO. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. VAPORESSO, the leading brand in the vaping industry, takes center stage at Vapexpo Paris 2024, presenting four of its latest vaping marvels: XROS, LUXE, ECO, and ARMOUR. These new innovative products garnered widespread acclaim from customers again, becoming the focal point of the event and further reinforcing VAPORESSO’s leadership in the vaping market. Renowned as one of Europe’s premier Vape Events, Vapexpo attracts enthusiasts, professionals, and industry leaders worldwide. Among all products on display, the spotlight gleams on the newly launched XROS 4 & XROS 4 MINI which feature enhanced flavor profiles and extended pod life with upgraded COREX 2.0 technology, aiming to provide users with the best vaping experience. COREX 2.0 technology has been meticulously refined from its first generation by optimizing morph mesh sizes for ideal temperature matching. This results in faster heating and explosively rich flavors. Additionally, upgraded cotton material efficiently supplies e-liquid, preventing burnt tastes and extending usage life. The Pulse mode ensures robust output support, maintaining consistent performance regardless of battery life. The XROS series also stands out for its customization capabilities. XROS 4 and XROS 4 MINI are all equipped with a 0.4Ω pod cartridge. The XROS 4 offers three output modes along with a display screen for enhanced user interaction, the XROS 4 Mini adds an airflow adjustment feature allowing users to tailor their vaping experience further. Both devices are compatible with the universal XROS pod platform. Moreover, these new offerings boast an all-aluminum unibody design that not only enhances durability but also provides a more comfortable grip. By focusing on technological and design innovations based on consumer feedback, VAPORESSO always aims to provide an unparalleled product experience. “Innovation is key to delivering superior user experiences,” emphasized Jimmy Hu, Vice President of VAPORESSO, highlighting the brand’s commitment to setting new standards in vaping and meeting consumer needs through continual improvement. About VAPORESSO VAPORESSO was created in 2015 and is dedicated to establishing a smoke-free world while raising the quality of life for its users. Based on its continuous innovation, strict quality control, and substantial commitment, VAPORESSO creates products that can fit all levels and styles of vapers. Experience the future of vaping with VAPORESSO, please visit: https://www.vaporesso.com/. The post Press Release: Revolutionary XROS 4 & XROS 4 MINI Unveiled by VAPORESSO at VAPEXPO Paris 2024 appeared first on Vaping360. View the original article
-
Utah became the fifth U.S. state with a PMTA registry law and the sixth with a flavored vape ban when Governor Spencer Cox signed bill SB 61 into law Wednesday. The law will take effect Jan. 1, 2025. PMTA registry bills are currently being debated in about two dozen other state legislatures. In Vermont, Virginia and Florida, registry bills have already been passed by state legislatures and sent to governors to sign or veto. The registry bills are written and promoted by tobacco giants Altria Group and R.J. Reynolds, which have lost significant cigarette sales revenue to unauthorized disposable vapes. The Utah bill, which easily passed both houses of the State Legislature in February, adds further roadblocks to vaping consumers and businesses in a state with an already-complicated set of vape product restrictions. The law will: Ban the sale of vape products in flavors other than tobacco and menthol Ban the sale of products which have not either received FDA marketing authorization, or have premarket tobacco applications (PMTAs) still under review by the FDA Ban the sale of all unauthorized synthetic nicotine products (even those with pending PMTAs) Require manufacturers to submit the names of products they intend to sell, with proof they meet state requirements and a $1,000 fee per product, to the state by Aug. 1 Create a list of state-approved products (a PMTA registry) by Oct. 1 Ban the sale of all vape products not on the state registry beginning Jan. 1, 2025 The FDA has authorized just seven vaping devices (and tobacco-flavored refills for them). All of the FDA-authorized vapes are made by three Big Tobacco companies: Altria (NJOY), Reynolds (Vuse) or Japan Tobacco (Logic). The FDA has not granted marketing permission for any open-system (refillable) products, bottled e-liquid, or any product with a non-tobacco flavor. The bill, its supporters say, will combat an “epidemic” of youth vaping in Utah. The bill’s sponsor, State Senator Jen Plumb—a medical doctor—claims she has personally seen Utah teenagers suffering from nicotine withdrawal in hospital emergency rooms. Utah already bans online vape purchases by private individuals, and imposes nicotine-strength limitations on products sold in the state. Alabama, Louisiana, Oklahoma and Wisconsin have also passed PMTA registry (or directory) laws. California, Massachusetts, New Jersey, New York and Rhode Island have banned the sale of most flavored vapes. The post Utah Governor Signs Flavor Ban and PMTA Registry Bill appeared first on Vaping360. View the original article
-
The FDA has asked the Supreme Court to review the Fifth Circuit Court’s decision against the agency in the Triton Distribution appeal. If the court accepts the FDA’s petition, it could forever alter the agency’s premarket review process—or it could validate the FDA’s current practices. In January, the Fifth Circuit ruled 10-6 for Triton in a rare en banc rehearing of the company’s MDO appeal. The decision overturned a 2022 decision by a three-judge panel of the same court. In the 2024 decision, Fifth Circuit Judge Andrew S. Oldham harshly criticized the FDA, referring to the premarket review process as a “wild goose chase.” Triton, an e-liquid manufacturer formally known as Wages & White Lion Investments, LLC, filed a petition for review of its FDA marketing denial order (MDO) on Oct. 6, 2021—one of the first companies to challenge a denial order. The Triton appeal was consolidated with sister company Vapetasia’s appeal that same month. Dozens of vape manufacturers have since challenged MDOs in federal courts. The petition for a writ of certiorari was filed today by U.S. Solicitor General Elizabeth Prelogar, the Justice Department’s supervisor of litigation, on behalf of the Department of Health and Human Services and the FDA. The Justice Department represents federal agencies in court. To be accepted for review, four of the nine Supreme Court justices must agree to grant the writ. The high court accepts very few cases each year—just two to four percent of the petitions submitted. But there has been serious speculation recently that the court could accept a vaping appeal soon. With three circuit courts now having ruled fully or partially against the FDA, enough of a “circuit split” exists that the Supreme Court is likely to want to step in and address the issues. Two other Supreme Court petitions were filed recently by Magellan Technology and Lotus Vaping Technologies. The FDA petition asks the court to hold those appeals pending resolution of the Triton case. In February, the Fifth Circuit granted a motion from R.J. Reynolds to stay proceedings in the consolidated appeal of MDOs for Vuse menthol refills (for Vuse Vibe, Solo and Alto models) “pending resolution of any further proceedings” in the Triton case, “including potential proceedings before the United States Supreme Court.” The post FDA Petitions Supreme Court to Review Triton Decision appeared first on Vaping360. View the original article
-

Florida Bill Banning Delta 8 Sent to DeSantis
Θðηα mάkαя опубликовал тема в Новости электронного парения
Delta 8 and other hemp-derived THC variants could be outlawed in Florida soon if Governor Ron DeSantis signs a bill recently passed by the state legislature. If signed, the new law will take effect on Oct. 1, and Florida will become the 25th state to ban or restrict the sale of delta 8 THC. The bill also bans hemp-derived cannabinoids HHC, delta 10 THC, THC-O, THCP, and THCV, and all synthetic cannabinoids. The proposed law sets allowable delta 9 THC limits for hemp-based products, and sets the minimum age to purchase hemp extracts to 21. It also prohibits products that could be confused with food items like snacks or candy, and bans hemp product packaging defined as “attractive to children.” Both houses of the Florida legislature passed SB 1698 on March 6, on a mostly bipartisan basis. The governor has not indicated whether he will sign the delta 8 ban. Supporters of the bill in the legislature mostly dismissed concerns voiced by delta 8 consumers that they would be forced to turn to the cannabis black market, according to the Florida Phoenix. Since the 2018 federal Farm Bill legalized production of hemp-based derivatives, the market for delta 8 and other hemp-derived cannabinoids has exploded. “That statement answers the question for you what these products are,” said State Representative Tommy Gregory. “They’re drugs. They’re recreational drugs. And yes, if we say if you can’t buy them, and you’re a drug user, then sure, maybe you’ll go to a drug dealer. Maybe you’ll do the right thing and stop using drugs.” Delta 8 is a cannabinoid naturally found in cannabis plants—including both marijuana and hemp—but in concentrations too low to economically extract. Commercial delta 8 products are produced by converting legal hemp-derived CBD into delta 8. Since the 2018 federal Farm Bill legalized production of hemp-based derivatives, the market for delta 8 and other hemp-derived cannabinoids has exploded. Delta 8 and most other hemp-derived cannabinoids offer a milder high than marijuana-derived delta 9 THC. A ban on hemp-derived cannabinoids would affect many Florida businesses, including hemp growers, manufacturers and distributors, vape shops, convenience stores, and gas stations. Florida is the third-most populous U.S. state. The Florida Supreme Court is currently reviewing language in a proposed ballot initiative that would put recreational marijuana legalization to Florida voters this November. DeSantis has made clear that he is generally opposed to recreational legalization in Florida, although he concedes it is likely to happen. The court will decide on the ballot measure by April 1. The post Florida Bill Banning Delta 8 Sent to DeSantis appeared first on Vaping360. View the original article -

Vermont House Passes Flavored Vape/Tobacco Ban
Θðηα mάkαя опубликовал тема в Новости электронного парения
The Vermont House of Representatives today passed a bill that would ban flavored vape and tobacco products, and prohibit online sales of all nicotine products. The bill, Senate bill 18 (S 18), has already passed the state Senate. The bill will now go back to the Senate, where differences between the two versions will be ironed out. The bill will likely soon head to the desk of Governor Phil Scott to be signed into law or vetoed. If it becomes law, the flavor ban will take effect on Jan. 1, 2026. The Consumer Advocates for Smoke-free Alternatives Association (CASAA) issued a call to action for bill S 18 in April 2023, urging state residents to contact legislators and ask them to oppose the bill. The call to action is still active, and more important now than ever. If necessary, it will be updated to ask Gov. Scott to veto the bill. Vermont residents can also contact the governor’s office by phone at (802) 828-3333. Unlike the PMTA registry bills currently threatening vapers in over 20 states, the Vermont bill does not grant exemptions for products authorized by the FDA. Gov. Scott, a Republican, has expressed some concern over the tax revenue the state will lose if menthol cigarettes are banned. “We’re talking millions of millions of dollars, so if it’s $15 million we’re going to lose by putting this ban in place, I think we better reflect on that,” Scott said this week, according to NBC5. The Vermont bill, if it becomes law, will ban the sale of all flavored consumer nicotine products, including flavored vapes, nicotine pouches, smokeless tobacco and all forms of combustible tobacco, including menthol cigarettes. It will also ban online sales of all nicotine products, flavored or not. Unlike the PMTA registry bills currently threatening vapers in over 20 states, the Vermont bill does not grant exemptions for products authorized by the FDA. Five states currently have flavored vape bans: California, Massachusetts, New Jersey, New York, and Rhode Island. California’s law does not ban online sales of flavored vapes. California and Massachusetts are the only states so far to ban menthol cigarettes. Vermont currently imposes a 92 percent wholesale tax on vaping products—the highest rate rate in the country. The tax applies to products whether or not they contain nicotine. The post Vermont House Passes Flavored Vape/Tobacco Ban appeared first on Vaping360. View the original article -
NOTE: This is a sponsored press release written by VOOPOO. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. VOOPOO, a leading innovator in the vaping industry, is delighted to introduce two new additions to its esteemed ARGUS Pod series – ARGUS P2 and ARGUS G2. The two new super pods come with a 3X upgrade, fully upgrading the user’s vaping experience. ARGUS P2 and ARGUS G2 are the second generation of VOOPOO’s flagship pods, ARGUS P1 and ARGUS G. Compared with the predecessors, the new two products feature higher power and larger screens. And new cartridge upgrades the large capacity from 2 ml to 3 ml, achieves lower new 0.4Ω coil resistance, and upgrades side-filling to a convenient top-filling. These two products come with upgraded quality without price increase. Not only that, they also carry a leading “3X Upgrade” performance. The “3X upgrade” means that the new ARGUS top fill cartridge achieves comprehensive upgrades in three aspects: leakage, longevity and flavor. It aims to break the vaping pain points and bring users a worry-free vaping experience: 5 Times Upgraded Lifespan: The coil lifespan of the new cartridges can last up to 90 mL e-liquid usage without burning from VOOPOO lab test. The material of the coil is upgraded with thermo-stable cotton to increase its lifespan. In addition, the structure of the heating wire has been optimized to achieve multiple refills of e-liquid without burning. The upgraded material and structure helps its 5 times better leak proof performance than other cartridge in the market. 3 Times Upgraded Leakproof: VOOPOO lab test shows that ARGUS top fill cartridge can remain non-leakage in a standstill state for up to 30 days.The new cartridge has improved the multiple air inlet design and increased the e-liquid locking capacity at the bottom of the cartridge from 0.1mL to 0.3mL. These features improve the anti-leakage performance significantly and reach 3 times longer lifespan than other cartridge in the market. Upgraded Flavor: The newly launched VOOPOO ARGUS top fill cartridge is constructed to deliver longer-lasting flavor and richer clouds. It supports the coil resistance as low as 0.4 Ω, which is lower than most cartridges, delivering superior vape flavor. Plus, it is also available in 0.7 Ω resistance to meet the needs of users from MTL to RDL. Moreover, it is equipped with the leading iCOSM Code to ensure the taste. 3X upgraded cartridge also enjoys compatibility. This upgraded cartridge is now compatible with all ARGUS Pod family devices, bringing the convenience and fun of compatibility. It’s time to upgrade your pod! Meet VOOPOO’s new super pods ARGUS P2 & G2 with 3X upgrade. From the first instant ignition technology to the new pod mod category, VOOPOO keeps taking steps toward its mission of upgrading. Now VOOPOO is embarking on a third revolution- the popularisation of Pods. It means launching new pods that maintain the high performance of pods but integrate the portability and affordability of disposable to provide a new vaping option to users. Stay tuned for more about VOOPOO! For more information, please check VOOPOO’s official website: https://www.voopoo.com/ WARNING: This product contains nicotine which is a highly addictive substance. The post Press Release: 3X Upgrade! Meet with VOOPOO’s New Super Pods ARGUS P2 & G2 appeared first on Vaping360. View the original article
-
European cargo airline Cargolux announced last week its fleet of Boeing 747s will no longer carry disposable vapes. The company said it made the decision for “ethical reasons.” Cargolux’s Italian subsidiary Cargolux Italia will also stop carrying the popular vape devices. The action could affect distribution of disposables in several European countries. Cargolux said in a press release the decision was made “in response to growing concern about the adverse effects of these products on both public health and the environment.” Cargolux Airlines International is the seventh-largest cargo-only airline in the world, and the largest based in Europe. The company has offices in over 50 countries, and delivers freight to more than 75 destinations, including 10 cities in the United States. It also operates and is part-owner of Italian carrier Cargolux Italia. Echoing claims made by anti-vaping groups and politicians, Cargolux says disposable vapes “pose a significant risk to human health, particularly for younger generations, given their targeted marketing with attractive flavors.” The company also said the lithium ion batteries in disposables are not recyclable, which is not true. France has begun the process of banning disposable vapes, and the British government says it intends to ban them too. “With this action, we hope to contribute to reducing the availability of these products on the market,” said Cargolux president and CEO Richard Forson. “As a responsible corporate citizen, Cargolux aims for this initiative to encourage other logistics operators to adopt similar measures.” The two largest air carriers in the world, U.S.-based Fedex and UPS, both announced in early 2021 they would stop carrying vaping products (by air or ground) in the United States. The decision followed passage of a law that added vaping products to the Prevent All Cigarette Trafficking (PACT Act), banning U.S. Mail delivery of vaping products. The post Freight Airline Cargolux Will Stop Carrying Disposable Vapes appeared first on Vaping360. View the original article
-
The Massachusetts Supreme Court has upheld a so-called “tobacco-free generation” law passed in 2020 by the city of Brookline, Mass. The law bans the sale of vaping and tobacco products in Brookline to anyone born on or after Jan. 1, 2000. Unlike any other kind of tobacco or vape ban, a generational ban like Brookline’s allows some adults to legally buy tobacco or vapes while others are prohibited from doing so. A current 24-year-old born on Dec. 31, 1999, can legally buy a vaping product in the Boston suburb, but a 24-year-old born a day later, on Jan. 1, 2000, is prohibited from buying that same product—and always will be. While these bans are usually called “tobacco-free generation” or “smokefree generation” laws—because they invariably ban sales of cigarettes and combustible tobacco products—the Brookline ban has been referred to as a “nicotine-free generation” law, because it bans sales of all consumer nicotine products, including vapes. Court: cities may completely prohibit vapes and tobacco Brookline passed the generational ban in November 2020, and the law took effect in July 2021, after undergoing legal review by the attorney general. The law was challenged by a gas station and convenience store owner who said the ban hurt his business, which is located just a block from Brookline’s border with Boston, where tobacco sales are legal to anyone 21 or older. Eventually, other c-store owners joined the suit. A superior court dismissed the lawsuit in 2022, and the businesses appealed to the state Supreme Court. The court heard oral arguments last November. Throughout the legal challenge, the city was represented pro bono by lawyers from the Public Health Advocacy Institute (PHAI) at the Northeastern University School of Law. PHAI’s Public Health and Tobacco Policy Center works as a partner to the state Department of Public Health’s tobacco control program. In its decision, handed down March 8, the Supreme Court upheld the lower court decision, and rejected the argument that the generational ban conflicts with existing state laws that set the legal age to buy tobacco and vaping products at 21. State law, said the court, allows local municipalities to completely ban tobacco sales if they so choose. The decision was cheered by tobacco control groups hoping the generational ban concept catches on elsewhere. Action on Smoking and Health (ASH) issued a press release calling the ruling “a watershed moment in the history of the tobacco wars.” The generational ban concept could spread While Brookline is believed to be the first government to have imposed a generational tobacco ban, the idea isn’t new. It was developed and named Tobacco-Free Generation in Singapore. And though Singapore has not yet passed its own tobacco-free generation law, a tobacco control group named Tobacco Free Generation International is headquartered there. New Zealand became the first country to pass a generational ban in 2022—although the law was abandoned before it took effect after a new government was elected last year. The New Zealand law did not restrict the sale of vapes. A proposed revision of Malaysia’s tobacco control laws introduced last year originally included a generational ban (which would have banned vaping products), but the age-based prohibition was dropped after the country’s attorney general determined it was unconstitutional. British Prime Minister Rishi Sunak announced last October that he would pursue a smokefree generation law in England patterned on the New Zealand law (no vape ban). The UK Parliament has yet to vote on the proposal. Scotland is considering its own generational ban. The post Mass. Supreme Court Upholds Brookline Generational Vape/Tobacco Ban appeared first on Vaping360. View the original article
-
Legislators in Florida and Virginia have passed PMTA registry (or directory) bills in both state houses, and the bills now await approval or veto from their state governors. If the bills are signed into law, thousands of popular vape products will be removed from store shelves in those states. The bills in Florida and Virginia, like similar bills pending in more than 20 other states, would require vape manufacturers and sellers to certify under penalty of perjury that their products have been authorized for sale by the FDA, or are currently undergoing premarket review by the agency. Registry bills: an unwelcome gift from Big Tobacco The bills were written and lobbied for by R.J. Reynolds and Altria Group—big tobacco companies seeking to protect declining sales of their cigarette brands and unpopular Vuse and NJOY vapes. Similar laws have already passed in Alabama, Louisiana, Oklahoma and Wisconsin. If the bills are signed into law in Florida and Virginia, it will create havoc for vapers in those states, create black markets, and force many people back to smoking. The FDA has authorized just seven vaping devices (and some tobacco-flavored refills)—all made by subsidiaries of Altria, R.J. Reynolds, or Japan Tobacco. Together those devices represent less than five percent of the convenience store vape market, and virtually none of the specialty vape market (vape shops and online sellers). Florida and Virginia residents: contact your governor! The Consumer Advocates for Smoke-free Alternatives Association (CASAA) has issued calls to action that allow residents of the two states to send a letter asking their governor to veto the bills. You can send CASAA’s prewritten letter, or modify it with your own personal story, which is preferable (or replace the prewritten letter completely with your own text). If you call, be polite. Tell the operator you’re calling to urge the governor to veto the bill because it will ban products you use to avoid smoking. If you’re able, add that you don’t support handing the small vaping industry to Big Tobacco. Florida Gov. DeSantis has been sympathetic to the vaping industry before, vetoing a flavor ban bill in 2020. He may do it again if Florida residents ask him to. In Virginia, Gov. Youngkin reportedly already has concerns with the registry bill. He too may respond favorably if he’s aware of strong consumer opposition. Florida – ask Gov. Ron DeSantis to veto H 1007 Write: use the CASAA Call to Action – remember to add your personal story! Call: (850) 717-9337 Virginia – ask Gov. Glenn Youngkin to veto SB 550/HB 1069 Write: use the CASAA Call to Action – remember to add your personal story! Call: (804) 786-2211 The post Will Florida and Virginia Governors Sign or Veto Big Tobacco Protection Bills? appeared first on Vaping360. View the original article
-
A group of Democratic U.S. senators, led by Illinois Sen. Dick Durbin, has sent letters to 22 major convenience store retailers and distributors threatening them with legal consequences for selling vaping products that have not received FDA authorization. The action was announced in a March 7 press release from Durbin—a longtime foe of vaping. The other signatories are Sens. Richard Blumenthal (CT), Sherrod Brown (OH), Bernie Sanders (VT), and Ron Wyden (OR). Recipients of the letters include retailers like 7-Eleven, Circle K, Wawa and Pilot. The senators’ goal is to pressure retailers to clear their shelves of popular disposable vapes, and sell only the six available vaping devices authorized by the FDA, and their tobacco-flavored refills. Selling only authorized vapes would be commercial suicide for the retailers. Those six devices and their refills—all produced by subsidiaries of big tobacco companies—together account for less than five percent of the convenience store vaping market. The senators join a weird alliance of interests at war against disposable vapes that includes the Campaign for Tobacco-Free Kids and Marlboro manufacturer Altria Group. “We write to draw your attention to distributor and retailer responsibilities under the Family Smoking Prevention and Tobacco Control Act,” the letters say, “and to specifically highlight apparent widespread violations of federal law prohibiting the sale and distribution of unauthorized tobacco products at convenience stores, gas stations, and other retail outlets across the nation. “Under the law, no tobacco product—including electronic nicotine delivery systems such as e-cigarettes or vaping devices, including those containing nicotine not made or derived from tobacco—may legally enter the market for sale without having first received authorization by the Food and Drug Administration (FDA) that the product is ‘appropriate for the protection of public health.’” The senators don’t explain that thousands of products not on the FDA-authorized list are still under review by the agency, and others have received stays in federal courts protecting them from FDA enforcement. Dozens of vape manufacturers have challenged FDA marketing denial orders (MDOs) in court, with many cases still pending. The senators join a weird alliance of interests at war against disposable vapes that includes the Campaign for Tobacco-Free Kids and Marlboro manufacturer Altria Group. Along with publicity-hungry politicians, disposables are under attack in the U.S. by the FDA, tobacco control organizations, major tobacco companies, and state legislators acting on behalf of tobacco companies. Disposable vapes began to gain popularity in 2020, after the FDA made flavored pod vapes an enforcement priority. Since then, the flavored disposable vape market has grown rapidly, threatening sales of big tobacco-owned vape brands like Vuse and NJOY—and now even threatening cigarette sales, the tobacco industry’s bread and butter. The post Senators Threaten C-Stores Selling Disposable Vapes appeared first on Vaping360. View the original article
-

UK Announces Plans for Steep New Vape Tax
Θðηα mάkαя опубликовал тема в Новости электронного парения
British finance minister Jeremy Hunt announced today that the UK will impose taxes on vaping products for the first time, beginning in two years. The tax, which the government says will reduce youth vaping, will likely lead to fewer smokers switching to vapes, and push some current vapers back to smoking. Although the tax will be subject to a public consultation, the government’s spring budget document lays out the current plan in detail, according to The Mirror. The budget says the tax will be £1 per 10 milliliters of zero-nicotine e-liquid, £2/10 mL for e-liquids containing from 0.1-10.9 mg/mL of nicotine, and £3/10 mL on products containing e-liquid in 11 mg/mL or greater strengths. Currently, vapes are subject to a 20 percent value added tax (VAT—a sales tax), like most consumer products. The new tax will be in addition to the 20 percent VAT. So a consumer who currently pays £5 for a 10 mL bottle (the maximum legal size) of e-liquid in 18 mg/mL nicotine strength would be charged an additional £3 tax plus the £1 VAT, for a total cost of £9 (about $11.45 U.S.)—a total tax rate of 44 percent. NNA: UK is “systematically dismantling world-leading policy” The plan was cheered by tobacco control groups, and by British American Tobacco, whose cigarettes and Vuse vapes are losing market share to disposable vapes. BAT CEO Tadeu Marroco told the Financial Times that his company “loves regulation.” The New Nicotine Alliance (NNA)—which advocates for nicotine consumers—said in a statement that it is “exasperated that the government is systematically dismantling world-leading policy which was an example to the rest of the world on how to utilise innovative harm reduction approaches to rapidly reduce the toll of smoking-related disease.” News that the government planned to tax vapes leaked in January. A tax had been among the potential actions included in a public consultation that launched after the government announced plans in October to propose a “smokefree generation” law. Vape taxes and restrictions = cigarette sales protection In late January, Prime Minister Rishi Sunak said the government intends to ban disposable vapes, restrict available vape flavors, impose “plain packaging” rules, limit how vapes are displayed in stores, and move forward on its generational tobacco ban. Sunak also said that nicotine-free vapes would, for the first time, fall under the same regulations as vapes that contain nicotine. Nearly fifty countries have some kind of vape tax. Most have a per-milliliter e-liquid tex—like the proposed UK tax—or base the tax on the wholesale cost of products. There is no federal tax in the United States, but 31 states, Washington, D.C., and Puerto Rico, impose their own taxes. Research shows that vaping product taxes increase cigarette sales and smoking. Vapes and cigarettes are economic substitutes, which means government actions that disadvantage one (like taxes and flavor bans) increase sales and use of the other—including among teenagers. The post UK Announces Plans for Steep New Vape Tax appeared first on Vaping360. View the original article -
NOTE: This is a sponsored press release written by Alt Pro Expo. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. Miami, FL – Alternative Products Expo, the leading vape and smoke shop B2B event in the US, is presented by Lightfire Distribution and set to transform Miami’s Wynwood District into a global stage from March 14-16, 2024. With more than 250 exhibitors, including key sponsors like Trinity Hemp, Happy Distro, Fume, ZETA, and many more, the expo promises an unparalleled opportunity for smoke shop owners to discover 2024’s trending products. This year, Alt Pro Expo elevates the attendee experience by implementing unique buyer programs designed to maximize engagement and opportunity. The expo’s $100K inventory giveaway and exclusive buyer flight voucher program underscore its commitment to supporting the growth of businesses within the industry. The event will kick off with an invite-only yacht afterparty sponsored by Mellow Fellow, blending business networking with Miami’s renowned nightlife. “Alt Pro Expo Miami is more than a trade show; it’s a celebration of the vibrant culture and innovation driving the alternative products industry” said Craig Corban, Marketing Director at Alternative Products Expo. “Set against the backdrop of Miami’s artistic Wynwood District, we’re creating a dynamic environment for industry leaders to connect, learn, and grow.” Attendees can expect a curated experience that includes exclusive show deals, product reveals, giveaways, and direct access to the latest innovations in vaping, hemp derivatives, CBD, smokeables, and everything in the smoke shop universe. The expo not only serves as a platform for showcasing new products but also as a catalyst for discussions on industry trends and growth strategies. For additional information on Alt Pro Expo Miami 2024 and to register for the event, visit altproexpo.com/tickets/. And for a limited time, readers of Vaping360 will get complimentary tickets if they use code VAPING360 to register. About Alt Pro Expo Alt Pro Expo is the premier trade show for the alternative products industry, bringing together leading vendors, buyers, and brands to showcase the latest in cannabis, CBD, vape, and other emerging market trends. With a focus on innovation, education, and networking, Alt Pro Expo provides a comprehensive platform for industry advancement. Contact: For booth sales inquire here For media inquiries, email media@altproexpo.com The post Press Release: Alt Pro Expo Miami 2024, Where Innovation Meets Culture in the Heart of Wynwood appeared first on Vaping360. View the original article
-
Germany has legalized possession and home cultivation of recreational marijuana, but will only allow sales through non-profit “social clubs” with limited membership. The Bundestag, Germany’s parliament, voted 407-226 today in favor of the legalization measure. The law, when it takes effect, will allow possession by adults (age 18 and over) of up to 25 grams of cannabis in public spaces, and up to 50 grams in private homes. Up to three plants can be grown in each household. Possession and use could be legal as early as April 1, although that date may be pushed back, according to Marijuana Moment. The bill now goes to the Bundesrat, the German legislative body that represents German states, and it could be referred to a mediation committee, which would delay final adoption of the law. Despite the good news, German cannabis enthusiasts shouldn’t expect a large legal marketplace anytime soon. The bill’s sponsors were forced to curtail plans for sales in licensed dispensaries and pharmacies because of European Union concerns, according to the BBC. The bill instead creates a plan for non-profit “cannabis social clubs” that will grow and distribute cannabis to a maximum of 500 members per club. These clubs could begin operations as early as July, depending on progress of the bill in the Bundesrat. According to Marijuana Moment, there are plans to introduce another bill that would establish pilot programs for commercial sales in some German cities. That legislation must first be reviewed by the EU’s European Commission. Conservatives in the Bundestag have said they will scrap legalization altogether if they take power in next year’s elections, according to the BBC. Germany will become the ninth country to legalize recreational cannabis use, and just the third European Union member (Luxembourg and Malta are the others). Canada, Georgia, Mexico, South Africa, Thailand and Uruguay have also legalized marijuana possession and use. Twenty-four U.S. states, the District of Columbia, and three territories have legal weed. The post Germany Becomes First Major European Country to Legalize Weed appeared first on Vaping360. View the original article
-
Analysis published last week by Barclays Research—the investment research arm of the multinational bank—suggests that U.S. tobacco giant Altria Group will fall short of its fiscal year 2024 earnings estimates unless falling cigarette sales can be revived with a crackdown on disposable vapes. Barclays forecasts Altria’s fiscal year 2024 cigarette shipping volumes to decline by 10 percent, and models a 2 percent decline in EBIT (earnings before interest and taxes, a common measure of profitability). “It is possible,” says Barclays, “that US cig vols improve if the FDA/DOJ are able to successfully clamp down on disposable e-cig growth. If this happens, US cig vols will improve and Altria would be able to meet its [earnings per share] guidance of $5.00-$5.15.” The FDA hasn’t authorized the sale of any modern disposable vapes, which have gained popularity in recent years, and compete directly with cigarettes in convenience stores and gas stations. Altria supports PMTA registry laws to boost cigarette sales Barclays’ insights explain Altria’s support for state bills that would create so-called PMTA registries (or directories), and ban the sale of vaping products that have not been either authorized by the FDA or have premarket tobacco applications (PMTAs) still under review by the agency. Some of the bills also allow the sale of products that have been denied by the FDA, but remain on the market due to federal court orders (like R.J. Reynolds’ Vuse menthol refills). More than two dozen PMTA registry bills have been introduced in state legislatures since January. The Consumer Advocates for Smoke-free Alternatives Association (CASAA) has issued calls to action for 21 registry bills, indicating they are gaining traction among lawmakers or hearings have been scheduled. New bills are being introduced almost daily. According to Gregory Conley, legislative and external affairs director for the American Vapor Manufacturers Association (AVM), Altria executives have spoken in favor of registry bills at legislative hearings in multiple states. Here in Kansas for the hearing on HB 2801. Yet another Altria executive is here to advocate for his stock holdings not to decline. Reminder: Altria is losing cigarette sales because adult smokers are switching to disposables. pic.twitter.com/E9H4sD4BGQ — Gregory Conley (@GregTHR) February 20, 2024 The bills are designed to tamp down on sales of popular disposable vapes and bottled e-liquid, both of which compete with Altria’s combustible cigarettes, including the Marlboro brand. Disposable vapes also compete with Altria’s NJOY e-cigarettes, but NJOY is a tiny player in the vape market (and in Altria’s earnings outlook). (Vuse manufacturer R.J. Reynolds—which also produces Newport and Camel cigarettes—also supports PMTA registry laws.) Alabama, Louisiana and Oklahoma have already passed registry laws, and currently maintain registries of products allowed for sale. Lawmakers in both Alabama and Oklahoma have introduced bills this year that would beef up enforcement of the laws. Both Altria and R.J. Reynolds have also taken legal action to kill their vape competition. Last October, Altria subsidiary NJOY filed a lawsuit in a federal district court against dozens of manufacturers, distributors and retailers of disposable vapes, including the Breeze, Elf Bar, Esco Bar, Flum, Juice Box, Lava Plus, Loon, Lost Mary, Mr. Fog and Puff Bar brands. NJOY asked the court to bar imports by the companies, and said it would “consider further litigation activity.” (In January, the court dismissed most of the lawsuit.) The FDA does its part to protect cigarettes The FDA Center for Tobacco Products (CTP) has engaged for years in a whack-a-mole war with independent vaping businesses, mostly manufacturers and sellers of e-liquid and disposable vapes. The agency has issued hundreds of warning letters, ordering manufacturers and retailers to remove products from the U.S. market, and has followed up to seek high-dollar “civil money penalties” (fines) from repeat offenders. In some instances, the FDA has enlisted help from the Department of Justice to shut down small vape businesses. Products have been seized by the FDA at airports, and the agency has ordered its import inspectors to detain shipments of Elf Bar and Esco Bar disposables without first inspecting them. The FDA has authorized just seven vape devices—all of them manufactured by companies owned by Altria (NJOY), Reynolds (Vuse) or Japan Tobacco (Logic). The agency hasn’t granted marketing permission to any open-system (refillable) products, including bottled e-liquid, or any vape product in a non-tobacco flavor. The post Financial Analyst: Disposable Vapes Killing Altria’s Cigarette Sales appeared first on Vaping360. View the original article
-
FDA Commissioner Robert Califf is engaged in a campaign to pressure the White House to approve a rule banning menthol cigarettes. According to a Politico story published today, Califf—who was appointed by President Joe Biden—-has asked friends and health experts to “press their White House contacts over the status of the long delayed policy.” The rule, if finalized, will ban U.S. sales of menthol cigarettes and flavored small cigars. Califf has championed the policy, claiming it will reduce youth smoking initiation and make it easier for adult smokers to quit. According to Politico, in addition to engaging outside assistance, Califf has “enlisted senior officials at the White House and the Health and Human Services Department to help advocate for the ban,” and personally lobbied senior Biden aides. Politico’s description of Califf’s “behind-the-scenes encouragement of outside pressure” as an “unconventional policymaking tactic” probably undersells the actions. It’s hard to imagine any occupant of the nation’s highest office reacting with anything less than extreme annoyance to backdoor lobbying by an appointed sub-cabinet agency official. Biden is already well aware of Califf’s position on the proposed ban. FDA’s menthol cigarette ban could fall to political pressure The FDA menthol cigarette rule has been on hold since early December 2023, when its expected date was changed from 2023 to March 2024 in the White House’s fall agenda of planned regulations. (The 2024 date is a tentative one, not a firm commitment.) The final rule was sent to the White House Office of Management and Budget (OMB) for review last October. The OMB Office of Information and Regulatory Affairs (OIRA) reviews all agency rules before they can be finalized and eventually implemented. Between October and December, OIRA held meetings with both opponents and proponents of the menthol cigarette rule before making a final decision. Opponents of the ban fear police enforcement actions in minority neighborhoods and the growth of illicit sales. Proponents say a menthol ban would improve public health, especially among black Americans, but dismiss fears of unwelcome police interactions and a loss of crucial votes among angry menthol smokers (not to mention loss of personal liberty and bodily autonomy). A menthol ban will turn a regulated market worth billions over to organized crime. In fact look at the states that have already banned (MA) the illegal market is growing & a repeal of the ban has been introduced. Bill H.2406 https://t.co/vNiHrZXuAU — DianeGoldstein (@dianemgoldstein) July 25, 2023 It was during the OIRA review period that White House staff may have been scared away from the menthol ban, after warnings about political fallout, including potentially depressed black voting in November 2024. Biden considers black voters key to his reelection chances. (Menthol cigarettes are favored by a large majority of black people who smoke.) “We’re now in a political season, and it’s only going to get tougher for them to do it,” Campaign for Tobacco-Free Kids CEO Yolonda Richardson told Politico. “All the delays are to the benefit of the tobacco industry. That’s just more time they have to keep them on the street, that much more time to addict kids.” But not many kids are smoking cigarettes anymore. Among middle and high school students responding to the National Youth Tobacco Survey in 2023, just 1.6 percent reported smoking cigarettes (as little as one puff) in the past 30 days—the third year in a row that number has remained under 2.0 percent. FDA refuses to authorize menthol vapes to help smokers The FDA announced its intention to ban menthol cigarettes in 2021—four years after President Trump’s FDA Commissioner Scott Gottlieb had included the possibility of a menthol ban in his “comprehensive” tobacco and nicotine plan. The agency issued a draft menthol cigarette rule in April 2022. Under Biden, the FDA also planned to resurrect a major pillar of Gottlieb’s plan: a rule mandating very low nicotine content in cigarettes. But it was menthol prohibition that got the most traction, largely because tobacco control groups have been advocating for a menthol cigarette ban since the FDA Center for Tobacco Products (CTP) came into existence in 2009. Gottlieb’s “comprehensive plan” also included the intention to authorize a variety of vaping products, which the former commissioner believed would be accepted as cigarette substitutes by many smokers. There seemed to be a belief at the FDA that menthol-flavored vapes could be a valuable tool to prevent menthol smokers from turning to the black market if their preferred cigarettes were banned. Even during the first year of Califf’s FDA leadership under Biden—as the agency issued marketing denials for most flavored vaping products—menthol vapes and e-liquids mostly escaped the ax and remained under review. Califf claims FDA is doing everything it can to help menthol smokers quit. Ok, then why hasn’t the FDA authorized a single menthol e-cigarette? FDA has taken no action to educate smokers about safer nicotine alternatives. https://t.co/LW4nU74reo — Guy Bentley (@gbentley1) April 28, 2022 That changed after Califf named ex-CDC official Brian King to lead the CTP. Since King’s appointment, the FDA has treated menthol vaping products the same as other flavors, denying them based on surveys showing youth primarily use flavored products (vapers of all ages do). The agency under Califf and King no longer seems to believe that menthol smokers faced with a ban need attractive vaping alternatives. Califf is a longtime foe of vaping. As Barack Obama’s last FDA commissioner, Califf oversaw the rollout of the 2016 Deeming Rule, which originally included an outright ban on flavored vape products. Califf later complained that the White House OMB had removed the ban from the final rule. Before his 2021 return to the FDA, Califf advocated for a ban on all vape flavors, and even suggested a prescription-only model for vaping products. “The regulatory trifecta,” Califf wrote in 2019, between his FDA stints, “would be to: 1) require the tobacco industry to lower the amount of nicotine in its products to subaddictive levels (if nicotine can be dialed up using irradiation and selective breeding, it can also be dialed down, even if the law forbids regulation that reduces the level to zero); 2) ban over-the-counter vaping products; and 3) support prescription vaping so that the 30 million current tobacco users do not go through acute withdrawal all at the same time.” The post Report: Califf Pressuring Biden to Pass Menthol Ban appeared first on Vaping360. View the original article
-
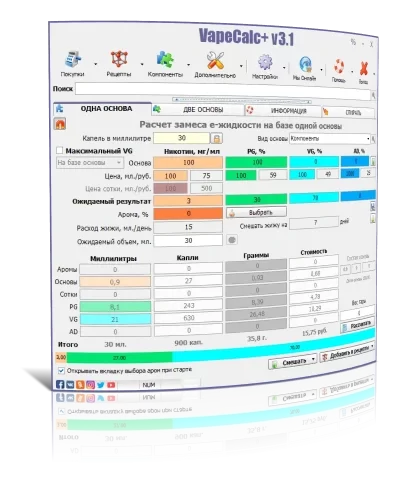
VapeCalc+ является отличным инструментом для всех вейперов и любителей электронных сигарет. Программа предлагает широкий спектр функций и возможностей, которые делают ее незаменимой для тех, кто хочет контролировать свои предпочтения в парении. Одним из главных преимуществ VapeCalc+ является простота использования. Интерфейс программы интуитивно понятен, и даже новичок сможет быстро разобраться в нем. Кроме того, программа предоставляет множество полезных опций, таких как расчет мощности, сопротивления, напряжения и других параметров, что позволяет пользователям настраивать свои устройства наиболее эффективно. Важной особенностью VapeCalc+ является возможность сохранять свои настройки и делиться ими с другими пользователями. Это позволяет вейперам обмениваться опытом и советами, а также сравнивать свои предпочтения с другими любителями пара. В целом, VapeCalc+ - это отличный помощник для всех, кто хочет получить максимум удовольствия от парения. Она помогает контролировать процесс и находить оптимальные настройки для каждого пользователя. Я определенно рекомендую эту программу всем, кто интересуется миром вейпинга.
-
VapeCalc + является отличным инструментом для всех вейперов и любителей электронных сигарет. Программа предлагает широкий спектр функций и возможностей, которые делают ее незаменимой для тех, кто хочет контролировать свои предпочтения в парении. Одним из главных преимуществ VapeCalc + является простота использования. Интерфейс программы интуитивно понятен, и даже новичок сможет быстро разобраться в нем. Кроме того, программа предоставляет множество полезных опций, таких как расчет мощности, сопротивления, напряжения и других параметров, что позволяет пользователям настраивать свои устройства наиболее эффективно. Важной особенностью VapeCalc + является возможность сохранять свои настройки и делиться ими с другими пользователями. Это позволяет вейперам обмениваться опытом и советами, а также сравнивать свои предпочтения с другими любителями пара. В целом, VapeCalc + - это отличный помощник для всех, кто хочет получить максимум удовольствия от парения. Она помогает контролировать процесс и находить оптимальные настройки для каждого пользователя. Я определенно рекомендую эту программу всем, кто интересуется миром вейпинга.
- 4 комментария
-
- обмен рецептами бесплатно
- база рецептов
- (и ещё 5 )
-
Two vape manufacturers are seeking Supreme Court review of their unsuccessful appeals of FDA marketing denial orders (MDOs). Both have filed petitions for writs of certiorari with the high court in recent weeks. The petitioners: Magellan Technology and Lotus Vaping Technologies Magellan Technology, Inc., filed its Supreme Court petition Jan. 22, asking the court to review its appeal denied last June 16 by the Second Circuit Court of Appeals. The appeal challenged MDOs issued in September 2021 for 12 non-tobacco-flavored refill pods for the company’s Juno pod vape. Lotus Vaping Technologies, LLC, petitioned the high court last Friday, Feb. 9 (in fact, so recently that the Supreme Court docket has not yet been updated with the filings). Lotus received MDOs in September 2021 for over 200 bottled e-liquids, representing several brands, numerous non-tobacco flavors, and multiple nicotine strengths. The company’s appeal (consolidated with Nude Nicotine, Inc.) was denied by the Ninth Circuit Court on July 7, 2023. Magellan is based in western New York State, and Lotus is located in Idaho. Both companies are represented by attorney Eric Heyer of the Washington, D.C.-based Thompson Hine law firm. Heyer has handled numerous appeals for vaping industry clients. Dozens of vaping manufacturers and distributors have challenged FDA denials in federal courts. Many cases are still pending. The circuit court split is growing To be accepted for review, four of the nine Supreme Court justices must agree to grant a writ of certiorari (Latin for “to be made certain”). The number of petitions accepted for review by the Supreme Court each year is small—only about two to four percent of those submitted. If the court does not accept the petitions, the lower court decisions will stand. So far, no vaping-related appeal has been granted a Supreme Court review. All the cases listed below were denied without comment. That said, some observers believe the FDA’s vaping regulations are ripe for review by the high court, primarily because the recent Fifth Circuit Court decision against the FDA in the Triton Distribution appeal indicates a stronger split in circuit court opinions than existed previously. Five circuits have upheld FDA denials. But the Fifth Circuit ruling, combined with an Eleventh Circuit decision in favor of six vape manufacturers in 2022, and a partial victory for Fontem US (Blu) last year in the District of Columbia Circuit, could be enough to convince four Supreme Court justices to accept the Magellan or Lotus appeal. The FDA could also seek Supreme Court review of its loss in the Fifth Circuit. This was a possibility mentioned by Case Western Reserve University School of Law professor Jonathan Adler in a Reason blog about the Triton decision. Adler, a renowned legal scholar, has written extensively about vaping regulations. Tobacco industry lawyers also seem to think the Supreme Court could step in soon. On Feb. 1, R.J. Reynolds filed a motion asking the Fifth Circuit to stay proceedings in its appeal of Vuse menthol refill MDOs “pending resolution of any further proceedings” in the Triton Distribution case, “including before the Supreme Court of the United States.” Previous vaping-related petitions to the Supreme Court As mentioned above, previous vaping petitioners have struck out so far at the Supreme Court: The court has rejected two cases brought by R.J. Reynolds that challenged flavor bans in California. On Jan. 8, the court declined to review Reynolds’ lawsuit against the State of California regarding the statewide law banning the in-person sale of flavored vape and tobacco products. In February 2023, the court refused to hear Reynolds’ challenge of a similar ban by Los Angeles County. Last October, the Supreme Court denied a petition by AVAIL Vapor for a review of its MDO appeal that had been denied in December 2021 by the Fourth Circuit. The petition was rejected without comment. In December 2021, the Supreme Court declined to hear Breeze Smoke’s application for a stay pending appeal of its MDO, after the Sixth Circuit Court of Appeals had already denied a stay. In June 2021, the high court denied a petition to rehear an appeal of a consolidated lawsuit by several small vape businesses challenging the FDA Deeming Rule. That case, Moose Jooce, et al. v. FDA, et al., had been filed and appealed by the Pacific Legal Foundation. Also in June 2021, the Supreme Court refused to hear a challenge to the Tobacco Control Act from Mississippi-based Big Time Vapes and the United States Vaping Association (USVA). The Tobacco Control Act is the 2009 legislation that granted the FDA authority to regulate tobacco and nicotine products. Big Time Vapes is believed to be the first vaping company to appeal a lower-court loss to the Supreme Court. The post Will the Supreme Court Hear a Vaping Appeal Soon? appeared first on Vaping360. View the original article
-
Already underway this week, and running from Feb. 5-10, the member nations of the Framework Convention on Tobacco Control (FCTC) are holding their 10th biennial meeting in Panama City, Panama. The FCTC is an international treaty, under sponsorship of the World Health Organization (WHO), and the member countries are called “Parties” to the treaty. The FCTC meeting this week is the 10th Conference of the Parties—or COP 10. COP 10 had been scheduled for November 2023, but was postponed, supposedly due to political unrest and violence. In fact, FCTC Parties haven’t met in person since COP 8 in 2018. Because of COVID, COP 9 was delayed until 2021 and then held virtually. Delegates agreed then to postpone discussions and decisions on many hot-button topics—including vaping and “novel” nicotine products—-until COP 10. Policy decisions made by COP attendees are binding on the member countries, and almost all the countries of the world—183 to be exact—are Parties to the FCTC. The Parties agree to conform their national laws and regulations to decisions voted by the whole FCTC, which means the COP meetings set tobacco and nicotine policy for most of the world. At COP 10, the FCTC Parties will make decisions about several major topics that could affect people who vape and use other non-combustible nicotine products. Some proposals, which are supported by the FCTC Secretariat (leadership group), include: Banning vaping flavors Banning open-system (refillable) products Banning disposable vapes Banning nicotine salt e-liquid Redefining the term “smoke” to include smoke-free vapor Regulating and taxing all nicotine products as harshly as cigarettes The WHO and its affiliates (like the FCTC leadership) have strongly opposed vaping and all consumer nicotine products almost since they were invented—and they’re not stopping now. Without opposition from major FCTC Parties like the UK, at least some of the proposals above will probably be adopted. Bloomberg, Tobacco-Free Kids and Dirty Ashtray awards The United States is not a party to the FCTC, and neither are Argentina, Cuba, Indonesia, Switzerland, and a handful of African countries. However, U.S. interests have outsized influence on the direction of the FCTC. Aside from the official U.S. delegation (which has no vote), many of the non-governmental groups granted “observer” status are American, as are their biggest funders, like billionaire ex-New York mayor Michael Bloomberg (who also funds the WHO’s tobacco control operations) and Microsoft tycoon Bill Gates. COP 10 is crawling with “observers” from Bloomberg-funded organizations dedicated to vaping restrictions and bans. These groups, like the Campaign for Tobacco-Free Kids (TFK) and Vital Strategies, set expectations for tobacco control policy direction, informing the prohibitionist FCTC Secretariat (leadership group), writing background documents and sometimes the policy proposals COP 10 delegates will vote on. Julio Ruades at @ElMonoVapeador, YouTuber and Spanish harm reduction activist, tried to conduct interviews for his channel at the #cop10fctc reception. He was kicked out of the building, leaving his half a million followers in the dark. Full version in comments! pic.twitter.com/CekNmHm4dJ — Vejpkollen (@vejpkollen) February 7, 2024 The Global Alliance for Tobacco Control (GATC)—an umbrella organization representing established tobacco control groups—acts as an organizing force and Greek chorus for tobacco control interests at the COP meetings. The GATC (formerly known as the Framework Convention Alliance) makes policy recommendations on proposed agenda items, and then praises or chides the delegates for supporting or opposing the GATC’s preferred positions, who are, of course, also the positions of TFK, Bloomberg Philanthropies and the WHO. The GATC publishes a daily COP bulletin that sometimes includes a “Dirty Ashtray award” that calls out what the GATC sees as unacceptable actions by delegates. Wednesday’s bulletin, for example, awarded the Dirty Ashtray to Guatemala for “setting a bad example by systematically timewasting and trying to reopen closed agenda items.” “Other parties take note,” they warned. Why should Guatemala’s delegates care what a bunch of private “observers” think? Because the GATC representatives’ reports will be considered carefully by the WHO and private funders when approving or denying grants for future projects that provide jobs and prestige. Countries and delegates could be punished for not “going along to get along” with the Bloomberg-controlled COP cops. Actual observers from the general public are excluded, and the delegates ritualistically vote on the first day of sessions to keep the press out—although there are some Bloomberg Philanthropies-approved (and -funded) journalists given better access to the event. FCTC COPs have all the trappings of a secret society, if not a full-blown cult. “Tobacco industry interference” means consumers are shut out Tobacco control observers and the FCTC leadership have a fear bordering on obsession about what they call “tobacco industry interference.” They appear to believe that COP delegates who wander away from the meeting venue will be accosted by lurking tobacco industry agents and corrupted. The fear betrays the tobacco control careerists’ lack of respect for the delegates. Their paranoia seems real—and often comical. “Tobacco industry operatives are crawling all over Panama City this week,” wrote Vital Strategies’ Jorge Alday in a tweet. “Their goal is to derail #COP10 where governments will meet to advance a global treaty that would reduce smoking.” We must continue advocating for urgent and accelerated implementation of the WHO FCTC. We must continue to be on our guard against the tobacco industry and its tactics.-@DrTedros #COP10FCTC pic.twitter.com/rgENYzhNqm — FCTCofficial (@FCTCofficial) February 5, 2024 In an essay in the Day 1 GATC bulletin, former Tobacco Control editor Ruth Malone wrote that “the single biggest obstacle to effective full implementation of the FCTC is tobacco industry interference, including at COPs.” She went on to provide examples, accusing the International Network of Nicotine Consumer Organisations (INNCO), British harm reduction advocates Knowledge•Action•Change, and INTERPOL (yes, the international police agency) of being tobacco industry moles who were denied access to previous COPs only through the vigilance of tobacco control purists. Those stories would be funny if the FCTC tobaccophobia didn’t ensure that legitimate representatives of nicotine consumers were excluded from the proceedings. Groups that advocate on behalf of vaping are completely shut out of all FCTC meetings, including COP 10. But, despite FCTC claims to the contrary, tobacco companies are present at COP 10. The call is coming from inside the house! The FCTC claims to exclude all tobacco industry representation, but that isn’t true. In fact, numerous member countries have ownership stakes in tobacco companies—including the largest tobacco company in the world. While mostly unpaid THR advocates like the nicotine consumer groups represented by INNCO are blithely accused of being tobacco infiltrators, the Chinese COP 10 delegation represents not just that country’s tobacco regulators, but also its tobacco industry. The State Tobacco Monopoly Administration (STMA) and the China National Tobacco Corporation—the largest cigarette manufacturer in the world— are run from the same building by the country’s Ministry of Industry and Information Technology, which has delegates in Panama this week. Other Parties to the FCTC that own or partly own tobacco companies include Egypt, India, Iran, Iraq, Japan, Lebanon, Malawi, Moldova, Syria, Thailand, Tunisia, and Vietnam. Most of these countries have banned or harshly restricted access to low-risk nicotine products like vapes—not to protect the health of citizens, but to guard national tobacco interests and tax revenue. “The effect,” writes Clive Bates, “is to create a bubble, an echo chamber of uncritical applause and a lack of diversity and experience in and around the COP meetings, supposedly done to prevent ‘tobacco industry interference’ while overlooking the presence of tobacco industry interests present on several delegations. The small consumer organisations are entirely excluded, yet they are the most directly affected and at-risk stakeholder group.” Resources for the COP-curious Watching the actual debates and deliberations is probably impossible, but you can sort of follow COP 10 proceedings as they happen. Here are some resources: COPWATCH – The COPWATCH website, run by vaping and tobacco harm reduction (THR) advocates, has done a good job following the FCTC, and provides updates during the day, explaining any news that comes from the COP 10 event. They also provide interesting links to news articles and other resources. GOOD COP (Conference of the People) live stream – The Taxpayers Protection Alliance is streaming live COP 10-related conversations from Panama on its YouTube page. Longtime THR advocates (and TPA employees) Lindsey Stroud and Martin Cullip, along with other TPA figures, have hosted a variety of interesting guests during their daily streams. (Recordings of previous discussions are also available to view.) Official FCTC COP 10 streaming – The FCTC has allowed some of the sessions to be streamed live from the official website (bottom of the page) this year. Don’t expect to see nitty-gritty details discussed, but the leadership has allowed some floor speeches from the delegates to be streamed. Global Alliance for Tobacco Control (GATC) daily bulletins – This bulletin doesn’t necessarily explain much of the goings-on at the event, but it will give you a look at the Bloomberg-funded tobacco control establishment’s take on the proceedings. Twitter/X – In addition to posts from the FCTC and the activist “observers” on site in Panama, there is plenty of COP 10 discussion among vaping and THR advocates. #COP10FCTC is the official COP 10 hashtag. The Global State of Tobacco Harm Reduction (GCTHR) COP 10 briefing paper – A deep dive into the COP 10 agenda by British harm reduction advocacy group Knowledge•Action•Change. GSTHR’s older FCTC explainer is also a must-read. Commentary on the Annotated COP 10 Agenda (Clive Bates) – This is a remarkable document, in which Bates comments on the official COP 10 agenda line-by-line, offering advice and criticism, and providing lots of valuable links. The post COP 10: Where Tobacco Control Plays for Keeps (With Your Life) appeared first on Vaping360. View the original article
-
SHENZHEN, China – UPENDS, an e-cigarette brand that prioritizes user experience and aims to become vapers’ favorite brand, launched its smart disposable flagship UPENDS STARX. The STARX features an industry-leading, cutting-edge atomization technology that represents a major breakthrough in atomization efficiency and vaping experience. In addition to the leap in performance, the STARX also redefined the industry design with a HD full touch screen display and dynamic light show, leading the development direction of disposable vapes and bringing the industry into the smart era. Amazing 20,000 puffs and attractive features makes STARX an annual flagship As UPENDS’ flagship of the year, the STARX comes with a built-in 18mL e-liquid that supports 20,000 puffs in PULSE mode and 10,000 puffs in SMOOTH mode. Its battery reaches 850mAh and is rechargeable with a Type-C cable. Users can intuitively monitor real-time e-liquid and battery levels and select vaping mode thanks to the HD full touch screen display, making it a ready-to-use vape. What’s more, the STARX allows users to adjust the inlet airflow in 3 levels for various vapor volume needs. A spaceship-like fashion item with full-screen display and flashing lights UPENDS STARX, which is built on the foundation of the product design experience and aesthetic accumulation, is not only a vape product, but also a futuristic fashion item. The STARX, which looks like a spaceship, boasts simple geometric, futuristic surfaces and smooth metalloid coatings that optimize the hand feeling. The full-screen display on the front adds to a sense of science fiction. In addition, as a fashion item, the STARX brings visual enjoyment and pleasure to users with its colorful flashing light rings on the screen. Smart mode switching powered by innovative heating tech UPENDS STARX is powered by its self-developed technology platform that intelligently controls the dual mesh coil to ensure the optimal atomization efficiency. “Unlike other dual mesh products, the STARX’s e-liquid storage cavity and entire airflow channel have been redesigned to keep the mesh coils in the best working condition,” said Dr. Wu, the CTO of UPENDS, “The STARX has passed rigorous tests in the lab, which makes it the top level of atomization efficiency among all products on the market, and fully meets users’ needs in terms of taste, flavor reproduction, vapor and puffs.” In addition, the STARX has two vaping modes, including PULSE mode, where two coils work simultaneously and SMOOTH mode, where two coils work alternately. Users can select the appropriate mode by tapping the touch screen 3 times. Stunning debut at Champs Trade Show and World Vape Show UPENDS STARX promises a fusion of cutting-edge technology, innovative design and user-centric philosophy, laying a good foundation for real smart vaping experience and brand new lifestyle. The STARX will make a stunning debut at Champs Trade Show, Las Vegas during February 14-17 and World Vape Show, Paraguay during March 8-9, showcasing the latest technologies and the best experience to every vaping enthusiast and lover. About UPENDS Founded in 2019, UPENDS is an e-cigarette brand specializing in electronic nicotine delivery systems. The excellent reputation of UPENDS as a leading e-cigarette brand is attributed to its innovative ability, superior manufacturing technology and quality, delicate design, and cost-effective features. UPENDS is currently expanding its global development. UPENDS constantly strives to prioritize the user experience and be the vaper’s favorite. For more information, please go to www.upends.com. Contacts UPENDS Marketing Team info@upends.com The post Press Release: UPENDS Launches Futuristic Disposable STARX To Spark the Real Smart Vaping Experience appeared first on Vaping360. View the original article
-
NOTE: This is a sponsored press release written by MECHA KING. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. [February 7, 2024] – Today, MECHA KING, a pioneer in cutting-edge vaping technology, proudly unveil its latest innovation – the Motron. With an emphasis on unparalleled vaping satisfaction, Motron introduces Turbo Mode, propelling users into an elevated realm of flavor and dense vapor. Motron’s Turbo Mode represents the epitome of intense vaping experiences. With higher wattage, users can expect 9000 quick and profound puffs, delivering rich flavors and dense vapor. The dual coils ensure a dual thrill, increasing vapor density and smoothness by 50%, flavor duration by 80%, and flavor consistency by 100%. However, Motron doesn’t stop at Turbo Mode; it’s all about customization. Switch seamlessly to Norm Mode for a gentler vaping experience, where normal wattage meets soft flavor, allowing for more extended over 14000 relaxed puffs. Motron adapts to your preferences, ensuring each inhale is precisely tailored to your liking. Worried about power and e-liquid levels? Motron has you covered. The intelligent display on the Motron allows users to effortlessly monitor both battery and e-liquid levels. With this feature, users can enjoy a seamless vaping experience without any concerns about running out of power or e-liquid. Inspired by mecha aesthetics, Motron goes beyond being just a vaping device; it’s about embracing your distinct style. Motron’s design is a perfect blend of form and function, making it a statement piece for vapers who appreciate aesthetics as much as performance. In a world saturated with vaping options, Motron stands out as a beacon of innovation, offering a turbo-charged experience that caters to the diverse preferences of the vaping community. Motron is now available for pre-order at leading retailers and online platforms in USA. Join us in this sensory revolution and elevate your vaping experience to new heights with Motron. Parameters: E-juice Capacity: 16ml Battery Capacity: 650mAh Coil: Dual Mesh Coil Turbo Mode: 9000 Puffs Norm Mode: 14000 Puffs Flavors:12 For more information on Motron and its features, please visit www.mechaking.com For media inquiries and product reviews, please contact: info@mechaking.com About Mecha King: Mecha King is a leading innovator in the vaping industry, dedicated to providing cutting-edge technology and a diverse range of products that transcend the ordinary. With a fusion of design, technology, and passion, Mecha King continues to push the boundaries of what is possible in the world of vaping. The post Press Release: Unleash the Power of Turbo Mode with MECHA KING Motron, a Vaping Experience Like Never Before appeared first on Vaping360. View the original article
-
NOTE: This is a sponsored press release written by SKE VAPE. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. Shenzhen SKE Technology, a trailblazer in the vaping industry for over a decade, proudly introduces its latest innovation, MEMERS VAPE. Established in 2013, Shenzhen SKE Technology has consistently led the way in vape technology, garnering widespread acclaim and a robust user base across 70 countries. In 2024, Shenzhen SKE Technology continues its forward march, introducing MEMERS VAPE under its multi-brand strategy. This new brand epitomises the company’s commitment to cutting-edge technology and innovation, aiming to reshape market trends and meet evolving consumer demands. MEMERS VAPE, positioned as the “advocate and innovator of electronic atomization technology and application,” focuses on pioneering new technologies and innovations. It aims to spread the message of smoking cessation through its advanced electronic atomization products, targeting both smokers and e-cigarette enthusiasts. The essence of MEMERS is inspired by the internet’s “MEME” culture, reflecting enjoyment, creativity, and social engagement. This brand concept resonates with contemporary popular culture, emphasising individuality and shared experiences. MEMERS VAPE is not just an e-cigarette brand; it’s a cultural bridge connecting diverse individuals. MEMERS introduces “MITS Ecosystem Platform ” – a Miniaturised Integrated Technology System Ecosystem Platform. This innovative platform, soon to be launched, focuses on integrating various technologies into a compact system. It features high integration, rapid heating, consistent flavour, and incorporates big data, IoT modules, and an AI platform “HiSMK”, the world’s first AI platform in the e-cigarette industry which will be launched by Shenzhen SKE Technology. “HiSMK” is a globally pioneering AI knowledge graph in the electronic cigarette industry developed by Shenzhen SKE Technology. It aims to become an ecosystem platform leading the industry’s development. This platform uses AI as the underlying technology support, connecting and interacting with users, engaging in e-cigarette cultural exchange and services, utilizing AI big models to continuously evolve consumer demands, and ultimately realizing the full chain functionality of I2C (consumer ideas to consumers). MEMERS is set to launch a series of innovative products in the first and second quarters of 2024, including disposable, open-pod, and closed-pod systems. These products, especially those with AI technology, are poised to revolutionise the vaping experience. The MEMERS team, comprised of experts from leading companies worldwide, brings a wealth of global experience and a strong belief in the vaping industry’s potential. Adhering to the principle of “new technology & innovation,” they are ready to write a new chapter in vaping history. The post Press Release: Shenzhen SKE Technology Launches MEMERS VAPE, a New Chapter in Vaping Innovation appeared first on Vaping360. View the original article
-
NOTE: This is a sponsored press release written by AIR BAR. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. AIR BAR, a trailblazer in the disposable vape industry, proudly unveiled at this year’s Total Product Expo, its latest technological marvel: Precision Temperature Control (PTC) Technology. This revolutionary advancement is embedded in the highly anticipated Diamond Box, AIR BAR’s newest disposable vape device which received high demand at this year’s TPE trade show. The PTC Technology sets a new standard by intelligently adjusting atomization power in real-time, ensuring a continuous and stable vaporization experience throughout the product’s lifespan. R&D breakthrough: addressing industry pain points Disposable vape devices currently on the market often suffer from issues such as unstable flavor and easy burning. To tackle these challenges, AIR BAR has pioneered the use of Precision Temperature Control Technology. This cutting-edge approach, backed by an authorized invention patent (Patent No.: 2023118519017), monitors and controls the atomization core temperature, providing real-time adjustments for a seamless vaping experience. Precision Temperature Control Technology (PTC): elevating the vaping experience PTC operates by monitoring the atomization core temperature hundreds of times per second and adjusting the battery’s input power in milliseconds. This precision control ensures that the atomization core temperature stays within the optimal range for e-liquid, enhancing flavor and preventing issues like scorching. The technology spans the entire lifespan of the product, delivering a consistent and high-quality vaping experience. Advantages of PTC Technology: flavor enhancement, stability, and safety Flavor Enhancement: PTC ensures stable vaporization, maintaining the atomization core temperature within the optimal range (as shown in Table 1 below) for a superior flavor and vaping experience. Stable Flavor: The technology guarantees continuous and stable vaporization throughout the product’s lifespan, preventing the occurrence of a strong flavor followed by a fading taste. Scorching Prevention: PTC intelligently identifies low e-liquid levels and prevents scorching by adjusting or cutting off power output. Enhanced Safety: Real-time monitoring prevents issues such as overheating or excessive temperatures, ensuring user safety. The advantages of Precision Temperature Control Technology in disposable vape devices are mainly reflected in flavor optimization, flavor stability, scorching prevention, and improved safety. This ensures users a higher quality vaping experience. AIR BAR Diamond Box disposable vape device with PTC Technology AIR BAR unveiled Diamond Box, featuring Precision Temperature Control Technology, at Total Product Expo in Las Vegas, Nevada on January 31, 2024 at the Las Vegas Convention Center. The Diamond Box offers 20,000 puffs, a stunning 3D diamond-patterned body, OLED screen, and rechargeability via USB Type-C. With PHC (Pre-Heating Coil) and PTC technologies, the Diamond Box promises an unparalleled vaping experience. Diamond Box will next be available to experience at CHAMPS Trade Shows in Las Vegas held at the Las Vegas Convention Center on February 14, 2024 through February 17, 2024. AIR BAR, a proud continued sponsor of CHAMPS Trade Shows, looks forward to further educating both industry players and the public on its proprietary and revolutionary PTC technology. About AIR BAR Established in 2019, AIR BAR is a leading disposable vape company committed to pushing the boundaries of innovation. Dedicated to research and development, AIR BAR consistently delivers cutting-edge products, offering a healthier and safer alternative to smoking. For media inquiries: media@airbar.com The post Press Release: AIR BAR Introduces the Diamond Box Disposable with Precision Temperature Control Technology appeared first on Vaping360. View the original article
-

FDA Rejects PMTAs for Five More Blu Products
Θðηα mάkαя опубликовал тема в Новости электронного парения
The FDA has denied marketing applications for four Blu e-cigarettes and a menthol-flavored refill pod for the company’s myblu device. In January, the agency also rejected applications for Blu’s entire blu PLUS+ line of cartridge-based e-cigarettes. The products receiving marketing denial orders (MDOs) yesterday were: blu Disposable Menthol 2.4% blu Disposable Vanilla 2.4% blu Disposable Polar Mint 2.4% blu Disposable Cherry 2.4% myblu Menthol 1.2% The FDA said in a press release that Blu’s premarket tobacco applications (PMTAs) for the four blu Disposables and the menthol myblu pod “lacked sufficient evidence regarding harmful and potentially harmful ingredients in the aerosol for one product and battery safety for several products. Additionally, the applicant did not present sufficient data demonstrating that the new products have a potential to benefit adult smokers, in terms of complete switching or significant cigarette use reduction, that would outweigh the risk to youth.” The FDA decision eliminates the entire blu Disposable line except the Classic Tobacco-flavored device, which remains under FDA review. The blu Disposables are “cigalikes”—small, low-powered, cigarette-shaped vapes. No vaping products have been authorized since current FDA Center for Tobacco Products Director Brian King was appointed in July 2022. The menthol myblu pod denied yesterday is the last myblu product to be reviewed by the FDA and receive an MDO. However, the agency is conducting a second round of court-ordered reviews on the myblu device and tobacco-flavored refill pods (which received MDOs in 2022), after the the District of Columbia Circuit Court of Appeals ruled against the FDA on appeal. Blu owner Fontem US, LLC is likely to also challenge the new MDOs in court. Several dozen vape manufacturers have appealed MDOs in federal courts across the country. Since the FDA granted itself regulatory authority over vaping products in 2016, the agency has authorized just seven vaping devices, along with tobacco-flavored refills. No vaping products have been authorized since current FDA Center for Tobacco Products Director Brian King was appointed to the job in July 2022. All FDA-authorized products are made by Big Tobacco subsidiaries, and the agency has authorized no vaping products in non-tobacco flavors (including menthol), no open-system (refillable) products, and no bottled e-liquid. The post FDA Rejects PMTAs for Five More Blu Products appeared first on Vaping360. View the original article -
NOTE: This is a sponsored press release written by Rx Vape. The views and opinions expressed in this post are those of the authors and do not necessarily reflect the views and opinions of Vaping360. [Austin, TX, Feb 1, 2024] – Rx Vape, a trailblazer in the e-liquid market specializing in tobacco flavors, is actively seeking acquisition opportunities. With a solid foundation of over a decade in the industry, a dedicated customer base exceeding 50,000, and a reputation for unmatched quality and innovation, Rx Vape emerges as an attractive prospect for major tobacco companies and expansive vape businesses aiming to increase their market presence and diversify their product lines. Celebrated for its top-rated and extensively reviewed Virginia Tobacco flavor, Rx Vape shines as a model of excellence within the vaping community. Redefining the vaping experience Rx Vape’s pursuit of excellence goes beyond its comprehensive selection of proprietary tobacco e-liquids. The company is a pioneer in innovation, having recently unveiled nixamide/nixodine (Bonguard Naturals) – a revolutionary nicotine alternative sourced from natural plant elements. This cutting-edge product offers a vaping experience akin to nicotine, without sacrificing satisfaction, thus placing Rx Vape at the vanguard of changing consumer trends. A proven track record of success Rx Vape’s success story extends past its innovative products. With lifetime sales exceeding $10,000,000, the company has consistently offered exceptional value to its customers through a pure grassroots effort, relying solely on word-of-mouth marketing. Serving regions including Canada, Europe, Australia, parts of Asia, and the USA, Rx Vape’s steadfast commitment to quality and customer fulfillment has cemented its status as a trusted leader in the vaping domain. Navigating regulatory compliance: Rx Vape’s proactive approach In the ever-evolving landscape of the vaping industry, Rx Vape has shown a steadfast commitment to regulatory compliance, ensuring the enduring availability of its esteemed products. Collaborating closely with legal experts and industry bodies like the AVM (Association for Vaping Manufacturers), Rx Vape has meticulously prepared and submitted a comprehensive Pre-Market Tobacco Application (PMTA) to the FDA. This detailed PMTA, presently under FDA review, exemplifies Rx Vape’s commitment to transparency, regulatory compliance, and the perpetual enjoyment of their signature Virginia Tobacco flavor. Customer testimonials Harry Wexler, a longtime Marlboro smoker, expressed profound gratitude for Rx Vape’s Virginia Tobacco flavor, stating, “Please don’t ever get rid of this. I got off smoking Marlboro for 25 years with the Rx Virginia Tobacco. The only vape I found that still gave me the feeling and taste I would get from a real cigarette.” Martirag2000 echoed this sentiment, exclaiming, “Oh my gosh!!! The search is over!!! If you like Juul Virginia Tobacco, here it is. I cannot tell the difference. This is the closest I have ever bought. Please never go out of business.” Susan Smith, another satisfied customer, praised the authenticity of the flavor, declaring, “This is the best tasting tobacco flavor I have found. It is more like a real cigarette flavor. Not too sweet.” Unlocking growth opportunities The decision to explore acquisition options offers a unique opportunity for big tobacco firms and larger vape businesses to expand their influence and enhance their market position. An acquisition of Rx Vape provides immediate access to a diverse portfolio of proprietary tobacco flavors, valuable intellectual property, and an established distribution network. Such a strategic alliance offers a complete solution in the e-commerce domain, enabling the diversification of product offerings, capitalizing on the growing demand for electronic cigarettes, and securing a robust position in the evolving vaping landscape. For larger vape enterprises, acquiring Rx Vape means obtaining a respected brand, broadening their customer base, and leveraging Rx Vape’s expertise in product development and innovation, fueling their growth and propelling them to the forefront of the industry. Additional highlights Cultivating a customer-centric culture At Rx Vape, the culture revolves around listening to and acting upon customer feedback. This customer-centric approach has been instrumental in curating products that resonate with users, fostering a sense of community and loyalty. The company’s commitment to genuine, personalized customer interactions, especially through email communication, has played a crucial role in building its solid customer base. Adapting to industry trends with integrity While the e-liquid industry has seen shifts, particularly towards nic-salt vape juices and away from candy flavors due to regulatory changes, Rx Vape has remained true to its core mission: aiding smokers in their transition away from cigarettes. By focusing solely on tobacco flavors, Rx Vape offers a familiar alternative to smokers, aligning with its goal to support smoking cessation. Financial health and expansion potential With high profit margins and a growing market share, Rx Vape is on a trajectory of significant growth, which could be accelerated further with the right partnership. A potential acquisition promises to amplify Rx Vape’s brand, expanding its reach and influence in the vaping industry. A commitment to sustainability Rx Vape also emphasizes sustainability, offering refillable e-liquid bottles that drastically reduce waste compared to disposable vape pens. This environmentally friendly approach aligns with contemporary consumer values and market trends. Vision for future growth Rx Vape presents a robust platform for growth and expansion, with a well-established brand, industry expertise, and thoughtfully curated flavors. With an effective marketing strategy and expanded distribution network, the potential for exponential growth under new ownership is immense. Inquiries For more information or to express interest in the acquisition of Rx Vape, please contact Greg Cole at bd@rxvape.com. Website: www.rxvape.com The post Press Release: Rx Vape Seeks Acquisition – Pioneering E-Liquid Company Invites Industry Leaders to Partner for Growth appeared first on Vaping360. View the original article